Within just a year of their launch, India has administered over 2 billion doses of the COVID-19 vaccines. It is believed that CovishieldTM and India’s own, CovaxinTM, have prevented nearly 4.2 million deaths in the country. And yet, these vaccines are very different from the world’s first vaccine cleared for emergency use against the deadly virus: an mRNA vaccine.
Does India need a COVID-19 mRNA vaccine? To answer this question, I talked with Dr. Madhusudhana Rao (CEO of Atal Incubation Centre at the Centre for Cellular and Molecular Biology, Hyderabad) who spearheaded India’s foray into the COVID-19 mRNA vaccine world.
What is a COVID-19 mRNA vaccine? How is it different from CovishieldTM and CovaxinTM?
MR: mRNA vaccines introduce a piece of packaged SARS-COV-2 genetic code (the mRNA) into an individual’s body to elicit a protective immune response. On the other hand, CovishieldTM uses a harmless virus (adenovirus) integrated with SARS-COV-2 proteins, while CovaxinTM employs the whole inactivated SARS-COV-2 (incapable of causing disease) to deliver protection.
mRNA vaccines are touted as the next power tool in safeguarding health. They became very popular internationally in our fight against COVID-19 due to their short turnaround times of development. This means one has the flexibility to rapidly make and package a new mRNA against the evolving variants. This is not possible for CovishieldTM or CovaxinTM as they would require lengthy re-design and cell-culture durations. Additionally, since mRNA vaccines are completely synthetic, they can be manufactured in facilities that have space constraints as opposed to the aforementioned vaccines that need spacious biosafety facilities (areas enabling work with dangerous airborne pathogens).
In 2020, India did not have sufficient advances in mRNA vaccine technology. However, today we too can boast of having developed this skillset.
How was the idea of an indigenous mRNA vaccine born at AIC-CCMB?
MR: Right from the beginning of the COVID-19 pandemic, CCMB had the opportunity to play a role in its mitigation. Initially, it was involved in RT-PCR testing, which then evolved into viral genome sequencing. Eventually, we also worked on a couple of projects focused on sewage testing (as this can give an idea of how widespread the virus is in the community) and virus neutralization technologies (antibody-fragment based).
At the same time, COVID-19 mRNA vaccines had entered conversations abroad. Clinical trials showed they worked very well compared to any other platform and successively they got special emergency authorizations. In India, there has always been a lot of buzz around self-reliance, so we started questioning whether we could enter the sector of mRNA vaccine production as well.
Council for Scientific and Industrial Research (CSIR) was one of the first governmental agencies in the country to take interest in an indigenous mRNA vaccine. Although I drafted the project, we decided it should be a multi-institutional effort (involving IMTECH Chandigarh, NIIST Thiruvananthapuram, IGIB New Delhi, IICB Kolkata, IICT Hyderabad and IHBT Palampur) and it came through. Lastly, we stuck to the Moderna recipe, since it had more publicly available information, at that time.
It is now common knowledge that the Moderna vaccine comprises a lipid coat surrounding an mRNA core. How straightforward was it to follow their protocols?
MR: For guidance, we studied the patentsof Moderna and purchased most of the easily available components (such as commercial kits) off the shelf. The larger challenge manifested in the form of vaccine formulation, specifically the lipid nanoparticles (LNPs). Moderna employs specific equipment (syringes in a microfluidic set-up) manufactured in Canada for LNP production. When we approached the manufacturer, it proved difficult to obtain a quote. Later, when we received it, the exorbitant price floored us. This was a setback.
Fortunately, I have a lot of experience in lipid delivery. We figured out Moderna’s strategy from their publicly available videos, looked at relevant literature and designed a customized device. Mr. Gopi Suresh Oggu, a microfluidics expert and consultant, was crucial to our success. With this device, we were able to obtain LNP sizes similar to that of Moderna and similar efficacies as well.
Another challenge was the 5’ modification of mRNA also known as 5’ capping (mRNA capping is similar to wearing a hat on your head to protect it from harsh weather outside). A commercially available chemical cap is often attached to the mRNA. However, it is exceptionally cost-intensive, so we went for an alternate technology that makes use of enzymes of a popular virus called vaccinia for the same job.
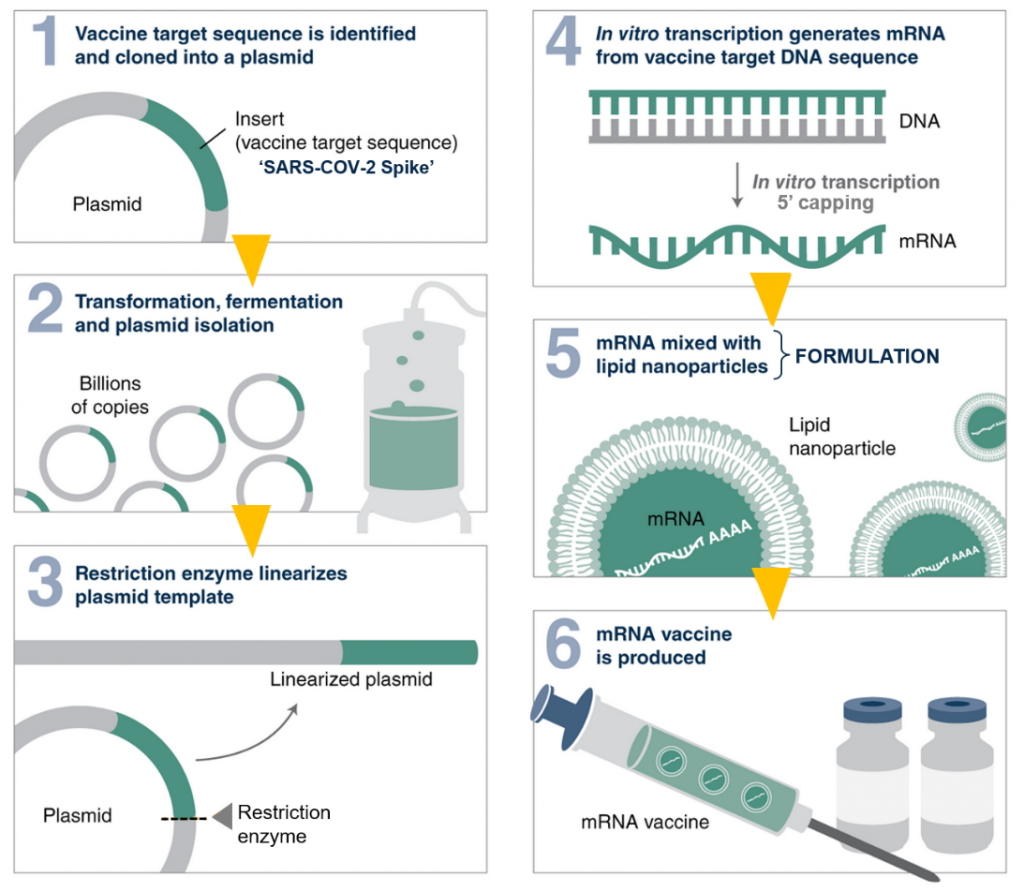
How did you proceed after vaccine production?
MR: After streamlining the formulation process, we tested the vaccine for antibody production. We performed standard experiments (0, 3 weeks, another booster) and quantified neutralizing antibody titers. Fortunately, CCMB also has a facility that houses the live SARS-COV-2 virus. We tested the ability of antibodies (generated by our vaccine) to neutralize the live virus and were rewarded with a successful outcome.
Simultaneously, we identified local manufacturers for modified nucleosides (mRNA), the 5’ caps and ionic lipids, easing our workload. The eventual vaccine particle performed well in all in vitro tests.
The next major step for the vaccine was animal testing, which we decided to do at the Indian Institute of Science, Bangalore. The results validated by an independent third party found that vaccinated animals were protected from coronavirus infection.
At the moment, we are awaiting the acquisition of this technology by an interested stakeholder.
Wish you the best for that! Do you feel the COVID-19 mRNA vaccines have changed the future of vaccine science?
MR: Definitely, there is no question about it! If you look up mRNA vaccine trials on the internet, you will come across hundreds of them. mRNA technology now has a foothold in several human diseases caused by other viruses, alleviating antimicrobial-resistant bacteria and even cancer. This is just the tip of the iceberg.


