In the rise of antimicrobial resistance among pathogens and infectious diseases, vaccines are a potent global health solution. Vaccines mimic the pathogens to train the immune system. The training allows the immune system to be quickly activated in the event of a real infection by the pathogen. Notable recent examples are vaccines against COVID-19, a disease caused by SARS-CoV-2 viral infection. While this was one of the greatest achievements in the history of vaccine development, the quest for vaccines against many pathogens has been challenging, resulting in little success. Why has that been the case? I will take you through one such example that I study; the Plasmodium parasites that cause malaria.
Despite a century-old battle to eradicate malaria, it remains one of the leading causes of global morbidity and mortality. WHO reported an estimated 247 million cases of malaria and more than 0.6 million malaria-associated deaths in the year 2021 in 84 malaria endemic countries. A majority of these cases are reported in countries of the African region. Within India, malaria incidence and deaths are higher in the states of Odisha, Chhattisgarh, Jharkhand, Meghalaya and Madhya Pradesh. The disease predominantly affects children under the age of 5 and pregnant women. Patients show symptoms such as irregular fever, chills, headache and malaise. Under severe disease conditions, complications arise, leading to renal failure, cerebral malaria, pulmonary oedema or haemorrhage.
The shape-shifting Plasmodium parasites
To date, more than 200 species of Plasmodium have been described, which cause malaria in a diverse range of vertebrate hosts. 5 strains are known to cause disease in humans. The parasites have a complex life-cycle, both within the mosquito vector, through which the disease is transmitted, and the human host.
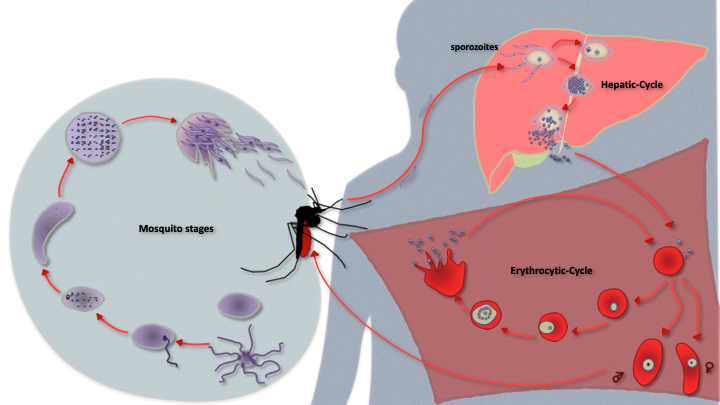
The parasite grows in female Anopheles mosquito and in humans, jumping between the two through mosquito bites. Within the human host, sporozoites navigate their way to the liver. They infect liver cells to form thousands of merozoites. These enter the blood stream, infect red blood cells (RBCs) and develop to give rise to ring, trophozoite and schizont forms. Mature schizonts rupture infected RBCs. This allows several merozoites to come out, which then infect fresh RBCs to continue the cycle. During this stage, some of the merozoites also get programmed to form male and female gametes, which are then picked up by mosquitoes.
Once in the mosquito’s mid-gut, the male and female gametes fuse to form a zygote. It matures to form a motile ookinete, which traverses the epithelial lining of the mosquito to form an oocyst. A single oocyst gives rise to close to a thousand sporozoites, which traverse to the salivary gland of the mosquito, and are now ready to find new hosts.
Those developing vaccines against the Plasmodium are interested in understanding this complex life-cycle of the parasite. That is because, there is a huge diversity in the proteins on the cell surfaces of the parasite during its developmental stages. And these proteins are the primary target of the immune system.
For example, PfEMP1 (P. falciparum erythrocytic membrane protein 1) is a protein expressed in at least 60 different combinations and targeted to the surface of parasite infected RBCs. While host immune system can identify one form of the protein, allowing it to clear these parasite infected RBCs, this immunity is lost when the parasites switch to express a different form of PfEMP1. These complexities make it challenging for the host immune system to keep a check on these enigmatic parasites, unable to target them at the right stage.
Malarial vaccines – One step at a time
Out of 133 malaria vaccines that have been in clinical development, two vaccines have been prequalified and recommended by the WHO – RTS,S/AS01E and R21/Matrix-M. We will focus on these two while others are still in trials or did not meet the WHO-set criteria.
Vaccine development requires identifying a part or whole of the pathogen, which will not cause an infection but help the immune system in identifying the pathogen quickly in case of a real infection. These are called as antigens.
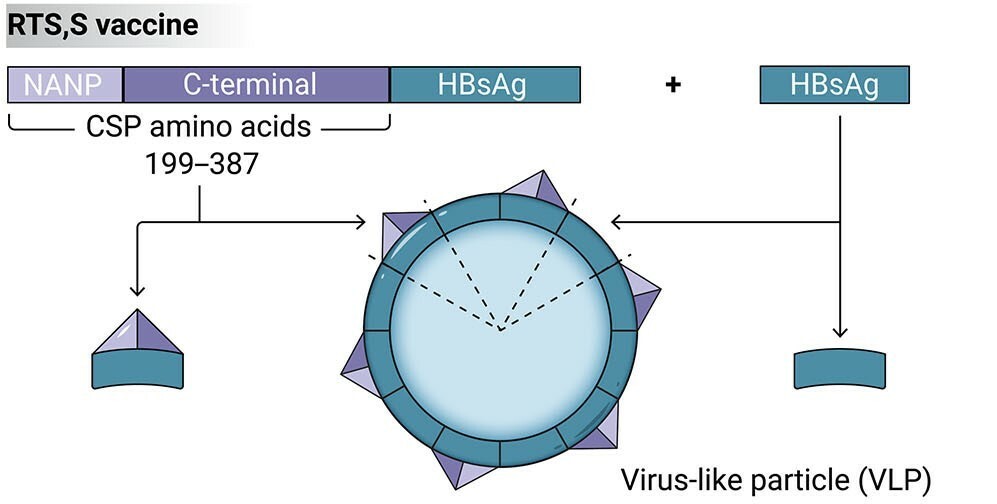
Initial efforts of malarial vaccine development used radiation-inactivated sporozoites. They don’t cause infection but still elicit immune responses. But this helped in achieving only partial protection. However, the researchers found that most of the antibodies produced through this vaccine was against the circumsporozoite protein (CSP), a major surface protein on sporozoites. This showed that the immune system acted the most against this protein, and CSP became the antigen of choice in malarial vaccine development.
The first effort was in collaboration between GlaxoSmithKline and Walter Reed Army Institute of Research, which resulted in the development of RTS, S vaccine. It is a designed like a virus. It contains 18 copies of a part of the CSP, called the repeat regions. These are attached to a hepatitis viral surface antigen, which helps in presenting CSP on virus-like particle’s surface. More access to CSP ensured better immune response.
This vaccine also contains adjuvants; substances that promote immune response, decrease the dose of antigen required and prolong the duration of protection. It uses a combination of liposomes and saponins (detergents) obtained from the Chilean soapbark tree, also known as Quillaja saponaria Molina, as its adjuvant.
Meeting the then WHO set goal of >50% efficacy, RTS, S/AS01 also known as MosquirixTM was the first to be approved in 2021 by the WHO for large scale production and trials.
Not all was sorted still. Clinical trials revealed that protection against malaria after administration of this vaccine is partial and wanes over time, leading to the recurrence of the malaria cases. We still needed vaccines with prolonged protection and improved efficacy.
Next came the R21/Matrix-MTM vaccine developed by Oxford University. Similar to the RTS, S vaccine, the R21 vaccine is also a virus-like particle, based on CSP. This, however, had reduced amount of viral antigen used in RTS, S vaccine.
This vaccine also used an advanced Matrix-MTM adjuvant. Developed by Novavax, it is composed of fractions obtained from the bark of Quillaja saponaria tree, mixed with cholesterol and phospholipids to give rise to unique nanostructures that enhance the host immune responses. It met the revised target of reaching >75% efficacy. And in 2023, WHO approved the vaccine for use in children aged 5 to 36 months.
The next obvious step is to produce and distribute the vaccine at a large scale. A cost-effective vaccine will be important for mass-scale vaccination in malaria prevalent countries of Africa. Serum Institute of India Pvt. Ltd. (SIIPL) has now been granted the licensure for production and distribution in Ghana. Current production capabilities at SIIPL are estimated to be over 200 million vaccine doses annually. They played an important role in developing affordable vaccines against many diseases, including COVID-19. And it remains to be seen if they transform malaria management.
Until then, mosquito bed nets, anti-mosquito sprays, rapid diagnostic tools for detection, and antimalarial therapies remain our most viable tools to use.